Our cells harbour a DNA detector termed cGAS to inform the immune system of viral and bacterial infections, cell death, as well as transformation during cancer. Researchers led by the Ikerbasque Research Professor Sergio P. Acebrón (University of the Basque Country and Heidelberg University) bioengineered this conserved cellular process into a novel fluorescent biosensor. The study, published in the flagship EMBO Journal, provides a multi-use biomedical tool to visualize the innate immune response to aberrant DNA across cell populations.
Each of our cells store their genetic information in the nucleus (genomic DNA) and mitochondria (mitochondrial DNA). A conserved molecular pathway controlled by the proteins cGAS, STING and IRF3 can detect DNA outside of these compartments, and report it to the neighbouring and immune cells by secondary messengers, including cGAMP and Interferon. As such, this system functions as a Swiss knife against cellular transformation and death, which often leads to host DNA by-products outside of the nucleus and/or mitochondria, as well as against foreign DNA from viral and bacterial infections. Interestingly, downregulation of these processes underlies viral and cancer immune evasion, while their aberrant upregulation is associated with auto-immune diseases. However, the lack of biological reporters to visualise these cellular processes have hindered research in the field.
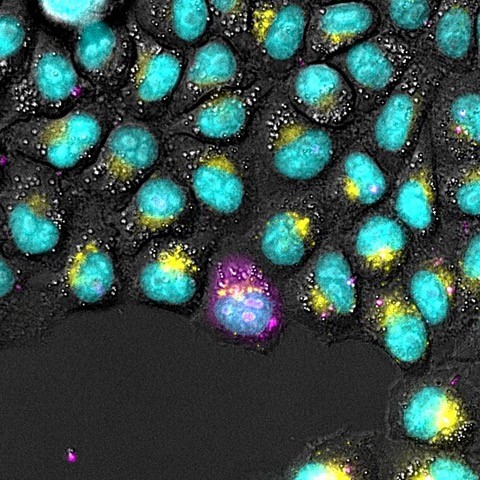
This study shows how engineering the functional interaction of activated STING and IRF3 can be used to capture the spatio-temporal and heterogenous dynamics of the response to the intracellular and extracellular messenger cGAMP. This novel fluorescent biosensor, together with imaging-analysis tools, allow the visualisation of single cell and population responses to Herpes virus infection, mitochondrial DNA release upon apoptosis, and other sources of aberrant DNA.
Tumour development is often associated with errors in segregating chromosomes that contain the genomic DNA, which can end up outside of the nucleus. The study shows that missegregated chromosomes do not activate the innate immune response through STING, likely due to the natural packing of the genomic DNA by histones. This is notable, as several clinical trials hoped to use STING as target against chromosomally unstable tumours.
This study represents a significant advancement in the study of the innate immune response by providing the community with a method to visualise it in complex biological models. This research has been funded by the Ikerbasque Foundation, Boehringer Ingelheim Fonds, and the excellence programme of Heidelberg University.
.png)
